Content

Lead-acid batteries are one of the oldest and most commonly used rechargeable battery technologies, dating back to their invention by Gaston Planté in 1859. These batteries are widely recognized for their affordability, high reliability, and ability to provide high surge currents, making them indispensable in various applications, including automotive, industrial backup, and energy storage systems. However, traditional lead-acid batteries have significant limitations, such as the need for frequent maintenance to replenish water lost during the charge cycle and the potential for hazardous gas emissions.
The introduction of Valve Regulated Lead Acid (VRLA) batteries represented a breakthrough in lead-acid battery technology. Unlike traditional flooded lead-acid batteries, VRLA batteries are sealed and maintenance-free, thanks to the incorporation of a valve-regulated system. This feature helps in preventing gas escape, allowing for gas recombination inside the battery. VRLA batteries are designed to address the shortcomings of conventional lead-acid batteries, offering improved safety, efficiency, and usability.
The purpose of this article is to explore the fundamental working principles of VRLA batteries, their structural components, classifications, and their advantages over traditional lead-acid batteries. Additionally, this paper will analyze the potential applications and future challenges facing VRLA technology.
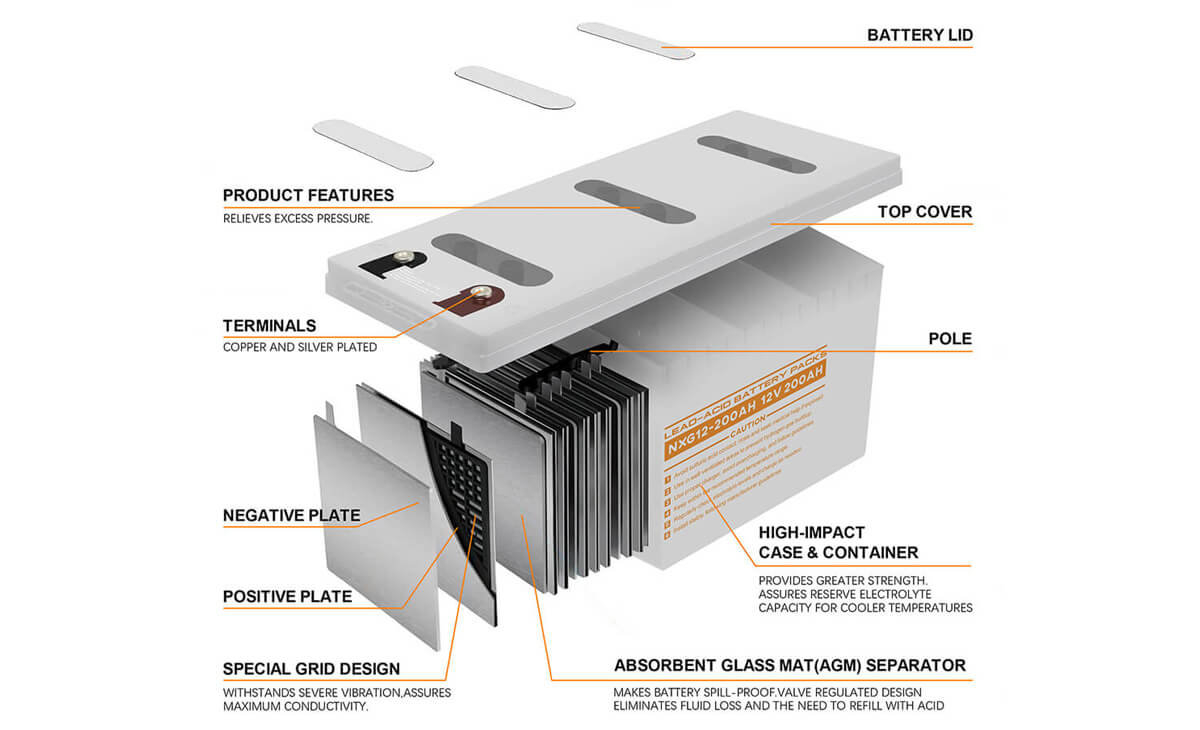
VRLA batteries operate on the same basic electrochemical principles as traditional lead-acid batteries, but they differ in design, which significantly improves their performance and safety. The key components of VRLA batteries include:
- Plates: The positive plate is made of lead dioxide (PbO₂), and the negative plate is made of sponge lead (Pb).
- Separator: A material, typically made of fiberglass (in AGM batteries), that keeps the positive and negative plates apart while allowing ionic flow.
- Electrolyte: The electrolyte is either absorbed in the separator or immobilized in a gel form, depending on the type of VRLA battery.
The most distinctive feature of VRLA batteries is their valve-regulated system. During charging, the chemical reactions inside the battery generate gases (hydrogen and oxygen). In traditional lead-acid batteries, these gases escape, leading to water loss and the need for maintenance. In VRLA batteries, the valve regulates the internal pressure by allowing excess gases to recombine within the battery, thus minimizing water loss. If the pressure exceeds a safe level, the valve opens to release excess gas and prevent damage to the battery.
The charging and discharging cycles in VRLA batteries follow the standard lead-acid reaction. During discharge, lead dioxide and sponge lead react with sulfuric acid, forming lead sulfate and releasing electrical energy. During charging, this process is reversed, regenerating the lead dioxide and sponge lead while consuming electrical energy.
VRLA batteries are mainly classified into two types: Absorbed Glass Mat (AGM) batteries and gel batteries. The table below outlines the key characteristics, advantages, disadvantages, and typical applications of these two types.
| Characteristic |
AGM Battery |
Gel Battery |
| Electrolyte Type |
Electrolyte is absorbed in fiberglass mat |
Electrolyte is immobilized in gel form |
| Charging Time |
Faster charging time |
Slower charging time |
| Vibration Resistance |
High resistance to vibration |
Moderate resistance |
| Performance in Hot Climates |
Less effective in high heat environments |
Performs better in hot climates |
| Cycle Life |
Typically shorter |
Longer cycle life, suitable for deep discharges |
| Overcharge Tolerance |
Sensitive to overcharging |
More tolerant to overcharging |
| Typical Applications |
Automotive, backup power (UPS), marine |
Solar energy storage, renewable energy, deep-cycle applications |
AGM Batteries:
AGM batteries are widely used in applications requiring high current, such as automotive starters, UPS systems, and marine applications. The fiberglass mat in AGM batteries tightly holds the electrolyte, allowing for more efficient conductivity and quicker charging. However, AGM batteries can be sensitive to overcharging, which may shorten their lifespan.
Gel Batteries:
Gel batteries, on the other hand, are known for their longevity and excellent performance in deep-discharge applications. The gelled electrolyte provides a more stable environment, making these batteries more resistant to extreme temperatures and overcharging. As a result, gel batteries are often used in solar energy storage, renewable energy systems, and other applications requiring frequent deep discharges.
Advantages:
- Maintenance-Free: One of the key advantages of VRLA batteries is their sealed design, which eliminates the need for periodic water refilling, making them maintenance-free.
- Sealed and Safe: Due to their valve-regulated system and sealed construction, VRLA batteries prevent gas leakage, ensuring safer operation compared to traditional flooded lead-acid batteries.
- Versatile Installation: VRLA batteries can be installed in various orientations without risking electrolyte leakage, making them suitable for confined spaces or locations with limited installation options.
- Low Self-Discharge: These batteries have a relatively low self-discharge rate, which means they can retain their charge for longer periods without needing to be recharged frequently.
Disadvantages:
- Lower Energy Density: Compared to modern battery technologies like lithium-ion, VRLA batteries have a lower energy density, meaning they store less energy for their size and weight.
- Shorter Lifespan: VRLA batteries typically have a shorter service life than lithium-ion batteries, especially when exposed to extreme temperatures or frequent deep discharge cycles.
- Overcharging and Deep Discharge Issues: VRLA batteries are sensitive to overcharging and deep discharging, both of which can significantly reduce their lifespan. Overcharging can cause excessive gas generation, leading to internal pressure buildup and potential damage.
Battery Management Systems (BMS): A well-designed Battery Management System (BMS) can help mitigate some of the disadvantages associated with VRLA batteries. A BMS monitors battery parameters like voltage, current, and temperature, preventing overcharging and deep discharge, and ensuring balanced charge distribution among cells. It can also provide real-time feedback on battery health, helping to extend the lifespan of VRLA batteries by optimizing their performance.
VRLA batteries are widely used in various industries due to their reliability and safety features. Below are some of the key application areas:
1. Uninterruptible Power Supplies (UPS):
VRLA batteries are commonly used in UPS systems to provide backup power in the event of grid failure. Their quick response time and maintenance-free operation make them ideal for critical systems in data centers, hospitals, and industrial facilities.
2. Electric Vehicles (EVs):
While lithium-ion batteries dominate the electric vehicle market, VRLA batteries are still used in smaller electric vehicles, such as golf carts and scooters, where their lower cost and simpler charging requirements are beneficial.
3. Solar Energy Storage Systems:
VRLA batteries are used in off-grid and hybrid solar energy storage systems to store excess energy generated by solar panels. Their deep-cycle capabilities make them suitable for providing continuous energy supply, especially in rural or remote areas.
4. Telecommunication Base Stations:
Telecommunication infrastructure, especially in remote areas, relies on VRLA batteries to ensure uninterrupted power for communication equipment. Their ability to operate in various orientations and confined spaces makes them ideal for such installations.
5. Marine and RV Applications:
VRLA batteries are often used in marine and recreational vehicles (RVs) for providing auxiliary power. Their sealed, leak-proof design ensures safe and reliable operation in environments prone to vibration and movement.
Typical cycle life comparison between VRLA batteries and other battery technologies:
This chart compares the typical cycle life of different battery types, highlighting the relative performance of VRLA batteries compared to more advanced technologies like lithium-ion.
Despite their widespread use, Valve Regulated Lead Acid (VRLA) batteries face several technical challenges that limit their performance and lifespan. The most prominent challenges include:
-
Limited Cycle Life: VRLA batteries have a shorter cycle life compared to lithium-ion and other advanced battery technologies. Repeated charge and discharge cycles degrade the lead-based plates, resulting in a gradual loss of capacity and efficiency over time.
-
Environmental Impact: The use of lead and sulfuric acid in VRLA batteries raises environmental concerns. While recycling systems for lead-acid batteries are well established, the process can still lead to hazardous emissions if not properly managed. Additionally, lead is toxic and poses health risks if improperly handled.
-
Material Improvements: The materials used in VRLA batteries, particularly the lead plates and the electrolyte, have inherent limitations in terms of energy density and durability. Improving the materials, such as developing lead-carbon composites or alternative alloys, could potentially extend the lifespan and performance of VRLA batteries.
Future Development Directions:
1. Electrolyte Formula Optimization: Advances in electrolyte chemistry could help reduce water loss and improve the recombination of hydrogen and oxygen, leading to increased efficiency. Researchers are also exploring the use of additives that could enhance the electrochemical stability of the electrolyte.
2. Valve-Regulated System Optimization: Improvements in valve technology can help better regulate gas buildup and release, reducing the risk of overpressure and enhancing safety. Additionally, advances in sensor technology could enable real-time monitoring of internal conditions, allowing for more precise control over the valve-regulated system.
3. Integration with Other Storage Technologies: While VRLA batteries have distinct advantages in terms of safety and cost, they face increasing competition from lithium-ion and other advanced battery technologies. Future developments may focus on hybrid systems that combine the benefits of both technologies, enabling more efficient energy storage solutions. For example, VRLA batteries could be paired with lithium-ion batteries in grid-scale storage systems to provide both short-term and long-term energy storage.
The global market for VRLA batteries remains robust, driven by their widespread use in industries such as telecommunications, uninterruptible power supplies (UPS), and renewable energy storage. Recent market data suggests that the VRLA battery market is expected to grow steadily, although at a slower rate compared to the lithium-ion battery market, which has seen rapid expansion due to the growth of electric vehicles and consumer electronics.
-
Market Size: According to market research, the global VRLA battery market is projected to grow at a compound annual growth rate (CAGR) of around 4-5% over the next five years. This growth is driven by increased demand for backup power solutions in data centers, hospitals, and commercial buildings, as well as the rising adoption of renewable energy systems.
-
Future Positioning: In the future, VRLA batteries are expected to maintain their stronghold in niche markets where safety, reliability, and cost-effectiveness are the primary concerns. For example, in telecommunication base stations and solar energy systems, VRLA batteries will likely continue to play a crucial role. However, their market share in high-energy applications, such as electric vehicles, is expected to diminish as lithium-ion technology continues to advance.
Comparison with Other Battery Technologies:
While VRLA batteries are cost-effective and reliable, they face stiff competition from lithium-ion batteries, which offer higher energy density, longer cycle life, and faster charging times. However, VRLA batteries will remain competitive in stationary energy storage and backup power applications due to their lower upfront costs and established safety record.
Valve Regulated Lead Acid (VRLA) batteries represent a significant advancement over traditional lead-acid batteries, offering a maintenance-free, sealed design that is both reliable and safe. Their ability to operate in various orientations, along with their low self-discharge rate, makes them an ideal solution for backup power, telecommunications, and renewable energy storage applications.
Despite these advantages, VRLA batteries face several challenges, including a relatively short cycle life, environmental concerns, and competition from more advanced battery technologies. To remain competitive, continued advancements in materials, valve-regulation systems, and hybrid energy storage solutions are essential.
Looking ahead, VRLA batteries are expected to maintain a strong presence in industries where cost and safety are paramount. However, as lithium-ion and other technologies continue to evolve, the role of VRLA batteries in the energy storage market may become more specialized. Nevertheless, with the right technological improvements, VRLA batteries could continue to provide a reliable and cost-effective energy storage solution for both industrial and consumer markets.







